Designing Efficient Horticulture Lighting Systems for Indoor Agriculture
- By Lumistrips LED Professional
- Feb 28, 2023
Industrial scale indoor agriculture under artificial lighting in closed and fully controlled environments could become the main factor that keeps at bay famine and related conflicts. With increasing population, diminishing area of agricultural land, pollution, global warming and migration to grow plants in a reliable, predictable and efficient way will become even more important in the future. For this reason it is important to understand and corectly apply the concepts of lighting for plant growth and development.
Concepts related to Horticulture lighting
A key factor in the success of indoor plant growth is the efficiency of the lighting system compared with sunlight, in the process of plant growth.
Plants grow via a process called Photosynthesis that converts electromagnetic radiation (light) into the chemical energy needed for growth and development. The other ingredients required are carbon dioxide (CO2), nutrients and water.
Photosynthesis and PAR radiation
The electromagnetic radiation required for Photosynthesis is defined as photosynthetically active radiation (PAR) and 400 to 700 nanometers has spectral range. Only radiation in this interval can be used by photosynthetic organisms in the process of photosynthesis, to fix the carbon in CO2 into carbohydrates.
Electromagnetic radiation called visible light or simply light for a typical human eye has a spectral range from about 380 to 740 nanometers.
A common unit of measurement for Photosynthetically active radiation PAR is the photosynthetic photon flux (PPF in short), measured in units of moles per second. For many practical applications this unit is extended to PPFD, units of moles per second per square meter.
The theory behind PPF is that every absorbed photon, regardless of its wavelength and energy, has an equal contribution to the photosynthetic process. As in accordance with the Stark-Einstein law, every photon (or quantum) that is absorbed will excite one electron, regardless of the photon’s energy, between 400 nm and 700 nm.
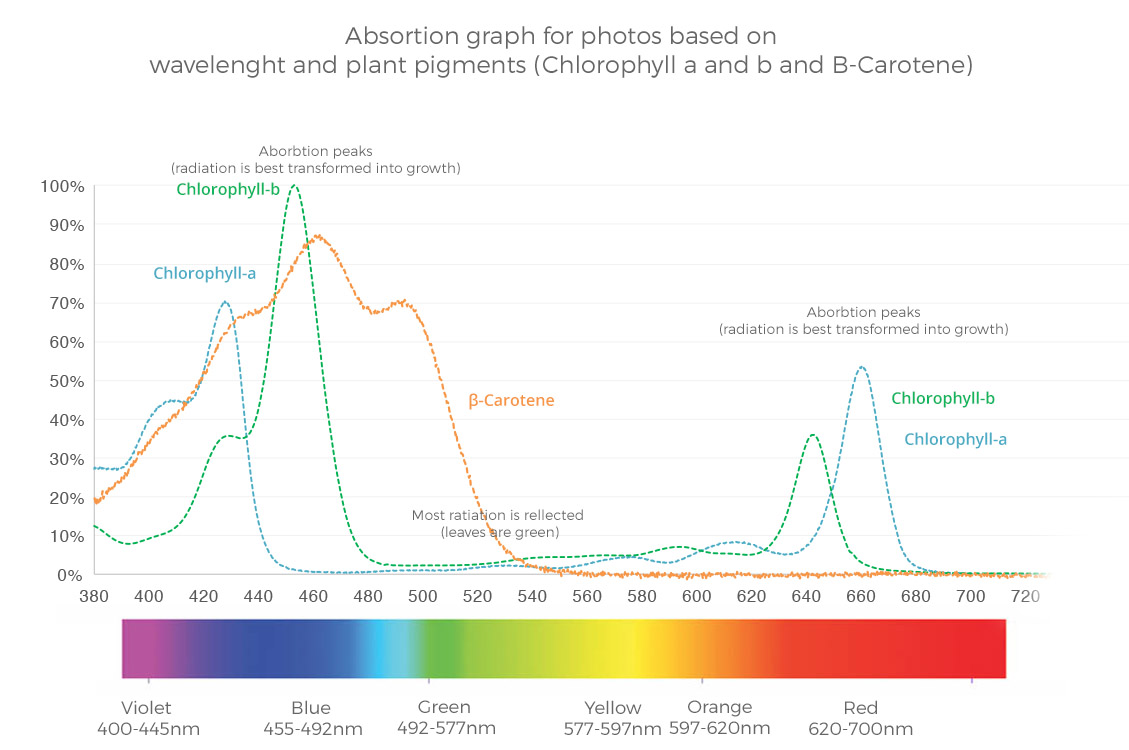
However, only some of photons are absorbed by a plant leaf, as determined by its optical properties and the concentration of plant pigments. The pigments are Chlorophyll A, Chlorophyll B, and Carotenoids (a/-Carotene, Lycopene, Xanthophyll).
The Chlorophylls A and B give plant leaves the characteristic green color because they reflect most of the radiation between 500 and 600 nanometres. Plants Where more Carotenoids than Chlorophylls are present plant leaves reflect wavelengths beyond 540nm and have yellow, orange, and red colors. This includes autumn leaves when Chlorophylls have dried away.
The graph below shows the typical absorptance spectra for Chlorophyll A, Chlorophyll B and Chlorophyll (beta-carotene). Each are explained briefly afterwards:

 Lumistrips UK
Lumistrips UK Lumistrips US
Lumistrips US Lumistrips ES
Lumistrips ES Lumistrips PT
Lumistrips PT Lumistrips ITA
Lumistrips ITA




